 |  |  |
|---|---|---|
 |  |  |
 |  |  |
PUBLICATIONS
β-Functionalized Zinc Porphyrin Coordinated to C60 Donor-Acceptor Conjugates
Two novel β-functionalized push-pull zinc porphyrins with amine or phenyl push groups and cyclic imide or carboxylic esters pull-groups have been newly synthesized for light energy harvesting applications. Donor-acceptor conjugates were subsequently built and characterized by coordinating an electron acceptor, C60Im, via metal-ligand axial coordination.
M. B. Thomas, R. G. Waruna Jinadasa, Y. Hu, B. Schmitz. H. Wang and F. D’Souza
“Special Issue for Gordon Research Conference”
Can. J. Chem., 2017. Just Accepted
β-Functionalized Trans- A2B2 Push-Pull Tetrabenzoporphyrins
β-Functionalized trans-A2B2 tetrabenzoporphyrins have been successfully designed and synthesized through newly developed regioselective bromination chemistry of porphyrins and a Pd(0) catalyzed three-step-one pot reaction. These benzoporphyrins exhibit broadened and bathochromic shifted UV-Vis absorptions and emission.
S. Kumar, X. Jiang, S. Qian, R. G. Waruna Jinadasa, K. Kadish, H. Wang
Chem. Comm., 2018, 54, pp 5303 - 5306
β-Functionalized Push-Pull Oppo-Dibenzoporphyrins as Sensitizers for Dye-Sensitized Solar Cells
A novel class of β-functionalized push–pull zinc opp-dibenzoporphyrins were designed, synthesized, and utilized as sensitizers for dye-sensitized solar cells. Spectral, electrochemical, and computational studies were systematically performed to evaluate their spectral coverage, redox behavior, and electronic structures.
Y. Hu, S. Yellappa, M. B. Thomas, R. G. W. Jinadasa, A. Matus, M. Shulman, F. D’Souza and Hong Wang
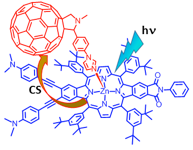
Competitive Energy and Electron Transfer in β-Functionalized Free-Base Porphyrin Zinc Porphyrin Dimer Axially Coordinated to C60
Simultaneous occurrence of energy and electron transfer events involving different acceptor sites in a newly assembled supramolecular triad comprised of covalently linked free-base porphyrin–zinc porphyrin dyad, H2P−ZnP axially coordinated to electron acceptor fullerene, has been successfully demonstrated. The dyad was connected through the β-pyrrole positions of the porphyrin macrocycle instead of the traditionally used meso-positions for better electronic communication.
Y. Hu, M. B. Thomas, R. G. Waruna Jinadasa, H. Wang and F. D’Souza
Highlighted in “Inside Cover Page”
Investigation of the Push-Pull Effects on β-Functionalized Benzoporphyrins Bearing Ethynylphenyl Bridge
A series of β-pyrrole functionalized push–pull porphyrins with amine push groups linked via an ethynylphenyl spacer, and cyclic imide or carboxylic esters as pull groups have been newly synthesized and characterized. The present findings bring out the importance of push–pull effects in governing the ground and excited (singlet and triplet) state properties of free-base porphyrins.
R. G. Waruna Jinadasa, M. B. Thomas, Y. Hu, F. D’Souza and H. Wang
Syntheses and PDT Activity of New Mono- and Di-Conjugated Derivatives of Chlorin e6
Syntheses of three new chlorin e6 conjugates for PDT of tumors are reported. One of the new compounds 17 is conjugated with lysine at the 13-1 position, but the others are mono-conjugated 14 or diconjugated 15 with the non-amino acid species ethanolamine.The most useful PDT photosentitizers appear to be the ethanolamine derivatives, conjugated at the 15-2 and the 13-1,15-2 positions; these show high phototoxicity but relatively low dark toxicity compared with 11, and also the highest dark/photo cytotoxicity ratios.
H. Chen, S. W. Humble, R. G. Waruna Jinadasa, Z. Zhou, A. L. Nguyen, M. G. H. Vicente and K. M. Smith
“Invited Article”
Monobenzoporphyrins as Sensitizers for Dye Sensitized Solar Cells: Observation of Significant Spacer-Group Effect
A series of monobenzoporphyrins (WH1–WH4) bearing different conjugated spacer groups were designed and synthesized as sensitizers for dye-sensitized solar cells. The monobenzoporphyrin bearing no spacer (WH1) resulted in a PCE of only 0.5 %; in contrast, the monobenzoporphyrin bearing vinyl spacers (WH4) achieved a PCE of 5.2 %.
R. G. Waruna Jinadasa, B. Li, B. Schmitz, S. Kumar, Y. Hu and H. Wang
Benzoporphyrins Bearing Pyridine or Pyridine-N-Oxide Anchoring Groups as Sensitizers for Dye-Sensitized Solar Cell
Novel benzoporphyrins bearing pyridine or pyridine-N-oxide groups were prepared through a concise method based on a Pd0 catalyzed cascade reaction. These benzoporphyrins were examined as sensitizers for dye-sensitized solar cells. Vicinal pyridine and vicinal pyridine-N-oxide groups were introduced as new types of anchoring/acceptor groups for dye-sensitized solar cells for the first time.
B. Schmitz, B. Li, R. G. Waruna Jinadasa, S. B. Lalvani, L. L. Kerr and H. Wang
“Invited Article”
Syntheses and Cellular Investigations of Di(aspartate) and Aspartate-lysine Chlorin e6 Conjugates
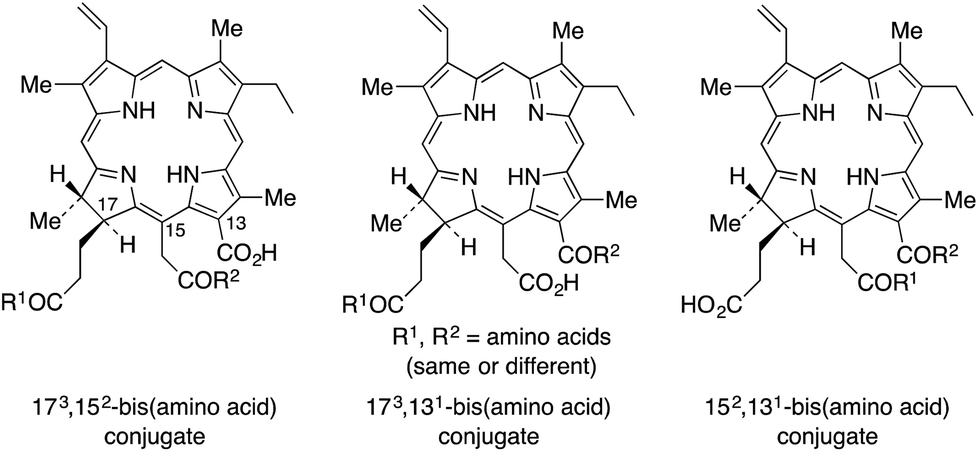
Chlorin e6 is a tricarboxylic acid degradation product of chlorophyll a. Four chlorin e6 bis(amino acid) conjugates were regioselectively synthesized bearing two aspartate conjugates in the 13-1,17-3 and 15-2,17-3 positions, or at the 13-1 ,15-2 via an ethylene diamine linker. One additional conjugate bearing two different amino acids, lysine at 13-1 via an ethylene diamine linker and an aspartate at 15-2 via a β-alanine linker was also synthesized. The cytotoxicity and uptake of four di(amino acid) chlorin e6 conjugates were investigated in human HEp2 cells, and compared with chlorin e6.
R. G. Waruna Jinadasa, Z. Zhou, M. G. H Vicente and K. M. Smith
Highlighted as a “Hot Paper”
β-Functionalized Push-Pull Oppo-Dibenzoporphyrins
The synthesis of a series of β-functionalized push–pull dibenzoporphyrins was realized. These porphyrins display subtle push–pull effects, demonstrating the exceptional tunability of their electronic and electrochemical properties.The structure–property studies provided in this work may provide useful guidelines for the design of new generations of materials in dye-sensitized solar cells, in nonlinear optical applications, as fluorescence probes, as well as sensitizers for photodynamic therapy.
R. G. Waruna Jinadasa, Y. Fang, S. Kumar, A. J. Osinski, X. Jiang, C. J. Ziegler, K. M. Kadish and H. Wang
Unsymmetrically Functionalized Benzoporphyrins
The synthesis of unsymmetrical push–pull benzoporphyrins has been realized. UV-Vis and fluorescence spectroscopy, cyclic voltammetry and DFT calculations reveal subtle substituent effects on the electronic and optical properties of these porphyrins.
R. G. Waruna Jinadasa, Y. Fang, Y. Deng, R. Deshpande, X. Jiang, K. M. Kadish and H. Wang
Chlorin e6 13-1:15-2Anhydride: A Key Intermediate in Conjugation Reactions of Chlorin e6
Since the patent for the photodynamic therapy agent Talaporfin (mono-L-aspartylchlorin e6) was issued in 1987, confusion has existed regarding which of the three carboxylic acid groups in the chlorophyll degradation product, chlorin e6 (1), is modified in standard amino acid type conjugations (using DCC or EDC and an organic base) with amino acids and other biomolecules. Here it is shown that the site of conjugation is the central 15-2 carboxylic acid, such reactions proceeding in numerous examples via a 13-1,15-2 anhydride for which a high resolution X-ray structure is reported.
H. Chen, R. G. Waruna Jinadasa, L. Jiao, F. R. Fronczek, A. L. Nguyen, K. M. Smith
Syntheses and Cellular Investigations of 17-3, 15-2 and 13-1 Amino Acid Derivatives of Chlorin e6
A series of amino acid conjugates of chlorin e6, containing lysine or aspartic acid residues in positions 17-3, 15-2, or 13-1 of the macrocycle were synthesized and investigated as photosensitizers for photodynamic therapy of tumors. The main determinant of biological efficacy appears to be the conjugation site, probably because of molecular conformation. Molecular modeling investigations reveal that the 17-3 substituted chlorin e6 conjugates are L-shaped, the 15-2 and 13-1 regioisomers assume extended conformations, and the 13-1 derivatives are nearly linear. It is hypothesized that the 13-1 aspartylchlorin e6 conjugate may be a more efficient photosensitizer for PDT than the commercial currently used 15-2 derivative.
R. G. Waruna Jinadasa, X. Hu, M. G. H. Vicente and K. M. Smith,








BOOK CHAPTER
This handbook will prove to be a modern authoritative treatise on the subject as it is a collection of up-to-date works by world-renowned experts in the field. Complete with hundreds of figures, tables and structural formulas, and thousands of literature citations, all researchers and graduate students in this field will find the Handbook of Porphyrin Science an essential, major reference source for many years to come.
H. Wang, R. G. Waruna Jinadasa, S. Kumar
Handbook of Porphyrin Science, Singapore, World Scientific Publishing Company, 2016 Sep; Pages 105-200.
“Invited Book Chapter”
CONFERENCES AND SYMPOSIA
Design, Syntheses and Characterization of Novel Chlorin Photosensitizers for Photodynamic Therapy
R. G. Waruna Jinadasa, X. Hu, K. M. Smith
Poster Presentation, 6th International Conference on Porphyrins and Phthalocyanines, San Ana Pueblo, NM, July 2010.
R. G. Waruna Jinadasa, X. Hu, K. M. Smith (Invited Talk)
Mini-Organic Symposium, Louisiana State University, Baton Rouge, LA, June 2011.
Design and synthesis of new π-extended porphyrins for solar energy conversion
R. G. Waruna Jinadasa, H. Wang
248th ACS National Meeting, San Francisco, CA, August 2014.
R. G. Waruna Jinadasa, Hong Wang, Bihong Li and Lei Kerr (Invited Talk)
229th ECS Meeting, San Diego, CA, May 2016.
Syntheses and Intracellular Localization of Chlorin-e6 Amino Acid Conjugates
K. M. Smith, R. G. W. Jinadasa, X. Hu, M. G. H. Vicente
7th International Conference on Porphyrins and Phthalocyanines, Jeju, Korea, July 2012.
Hong Wang, R. G. Waruna Jinadasa, Alex Matus, Shouzhong Zou, Lei Kerr
229th ECS Meeting, Chicago, IL, May 2015
Hong Wang, R. G. Waruna Jinadasa, Yuanyuan Fang, Siddhartha Kumar, Karl M. Kadish, Yi Hu
230th ECS Meeting, San Diego, CA, May 2016
Hong Wang, Lei Kerr, R. G. Waruna Jinadasa, Bihong Li, Benjamin Schmitz, Alex Matus, Yi Hu
230th ECS Meeting, San Diego, CA, May 2016
Y. Hu, R. G. Waruna Jinadasa, Hong Wang
Poster Presentation, 231st ECS Meeting, New Orleans, LA, May 2017
Hong Wang, R. G. Waruna Jinadasa, Michael Thomas, Yi Hu, Francis D'Souza
Poster Presentation, 231st ECS Meeting, New Orleans, LA, May 2017